Once upon a time, you could watch a BBC science documentary in the comfortable knowledge that it was written by scientists, and checked by scientists. The dialogue may have been presented by a famous suit, but the words would be kosher and relevant to the subject in question. All the visual stuff would also be relevant – no pointless fillers.
Now let’s skip forward a few decades to this article I saw on the BBC website today. And this is the World Service we’re talking about – another thing which was once considered an Oracle throughout the World. The title itself is bad enough:
Nitrogen: The bringer of life and death
This is misleading. Nitrogen itself is not poisonous, although that title would obviously imply that it is to any of the target audience. Humans need oxygen to survive, and it they don’t get it – in other words, breathe ANY pure inert gas that doesn’t have oxygen mixed in with it – they will suffocate and die. Even breathing pure oxygen for long periods is dangerous to most animals on earth.
Then there is the graphic, shown above. The introductory paragraph trumpets:
And yet this colourless, odourless gas, making up 78% of the atmosphere, has a highly explosive nature.
Nitrogen is not explosive – another conclusion the average reader will draw from this nonsense. It is relatively unreactive, though not actually inert, and it is its compounds which are explosive – and even then, only some of them (and not just because of nitrogen, either). The article mentions nitroglycerin and trinitrotoluene (TNT). The molecules of these two look like this: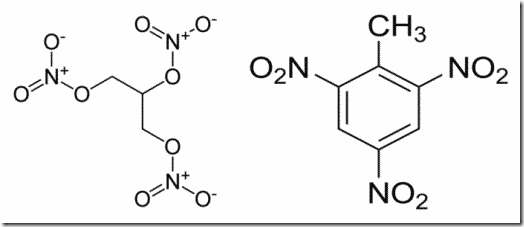
Years ago, this is what they would have shown you on any TV – or online, had it been available – show. But not any more. Nowadays, it’s puerile and inaccurate analogies that are used instead.
It’s worth pointing out that both od these molecules also contain carbon, oxygen, and hydrogen. In fact, nitrogen only accounts for about 19% of both in terms of their respective molar masses. Oxygen accounts for much more in nitroglycerin, and both oxygen and carbon account for more in TNT.
It is the extreme stability of nitrogen in its molecular state (N2) which partly accounts for the explosive nature of some of those compounds. In the case of TNT, other by-products include CO and CO2 and both of these are also stable. In the case of nitroglycerin, the -NO2 groups act as powerful oxidisers and by-products of combustion are N2, CO2, CO, H2, and O2 – the stability of all of these contributes to the explosive nature of the material. Going even further, the explosion of nitroglycerin is represented by this equation:
4 C3H5(ONO2)3(l) —> 12 CO2(g) + 10 H2O(g) + 6 N2(g) + O2(g)
There’s a lot more CO2 and H2 produced than there is N2. The article also refers to both TNT and nitroglycerin as “solids” when nitroglycerin is actually an oily liquid. Furthermore, compounds with nitro (-NO2) groups are rare in nature, but they do exist – and they’re not explosive.
The whole article leaps from one thing to another without properly explaining any of them. It talks as if nitrogen is the limiting element in plant growth, when in fact this role could equally be (and usually is) assigned to phosphorus. It just seems that the whole story begins with a premise and merely sets out to confirm that premise at all costs.